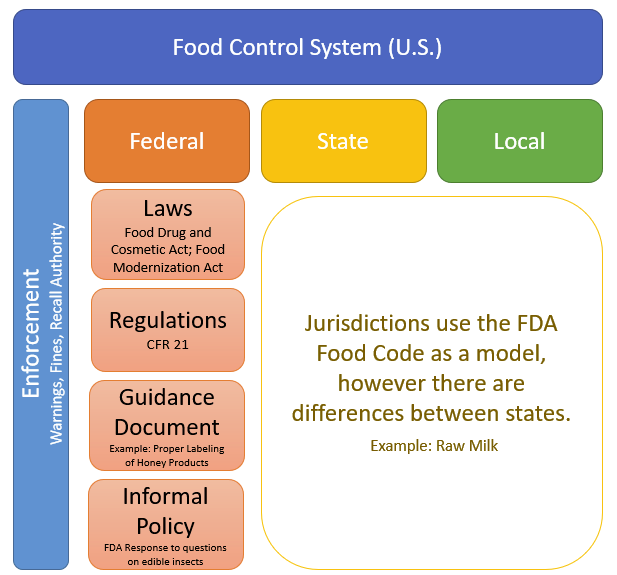
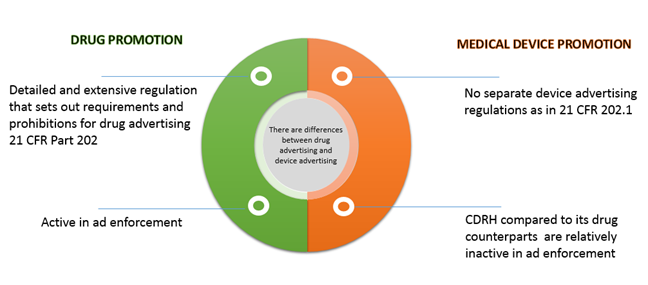
FDA CFR Part 11 Compliance | Security Assessment | Compliance Services | Certification & Attestation | DIY Platform

Current state of U.S. Food and Drug Administration regulation for cellular and gene therapy products: potential cures on the horizon - Cytotherapy

US FDA, EU GMPs, ICH Guideline, Japanese GMPs Handbook (Code of Federal Regulations) - Food And Drug Administration: 9781935131304 - AbeBooks

Book 23: 2023 Part 11 & Drug Development: Regulation, Preamble & FDA G – Clinical Research Resources, LLC

The Complete Code of Federal Regulations, Title 21, Food And Drugs, FDA Regulations, 2016 - Kindle edition by United States Government. Professional & Technical Kindle eBooks @ Amazon.com.
.jpg)
FDA CFR Title 21 Food and Drugs Regulations - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.